Stretch-Bend Transition of Water Clusters
an investigation of the two-quanta transition
I committed the final year and a half of my PhD to investigating the Stretch-Bend transition of various Water Clusters.
So what does that mean?
First, a water cluster is a small collection of water molecules in a specific arrangement. They can be either nuetral (all water molecules), positively charged (an extra hydrogen atom, usually in the form of H3O+), or negatively charged. In this project we looked at nuetral and positively charged water clusters. More specifically, the goal was to look at different hydrogen bonding environments. These environments are designated by their number of hydrogen-bond donors (D) and acceptors (A), some examples are shown below.
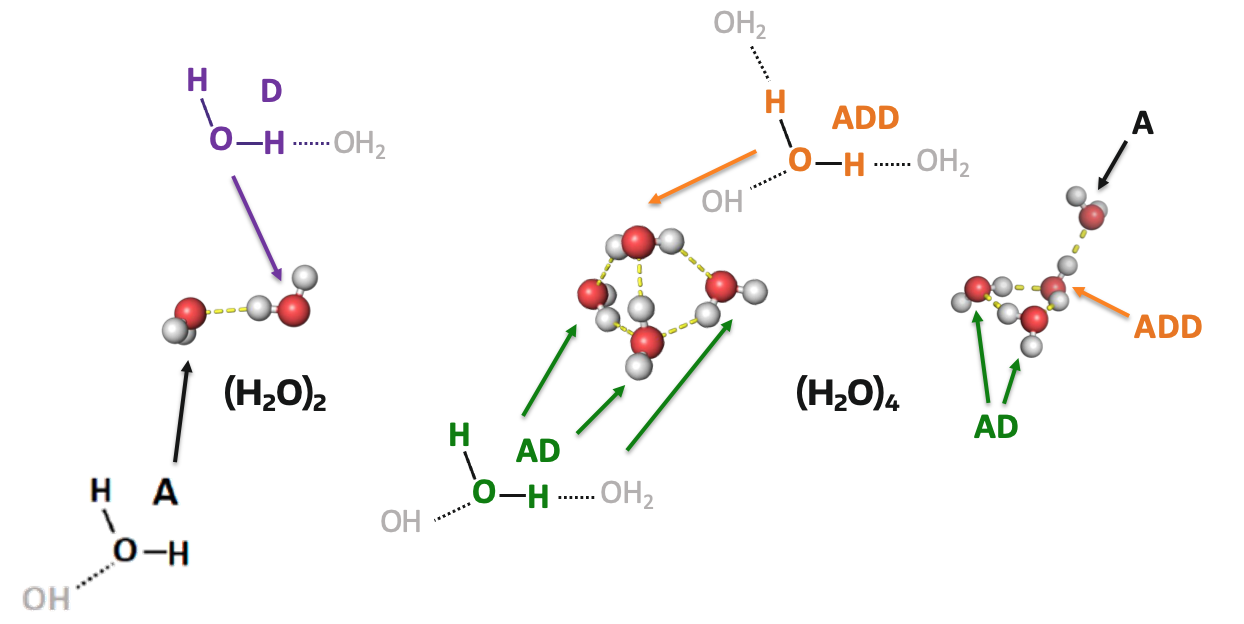
Various hydrogen-bonding environments shown in a water dimer and water tetramer cage and three-one (left to right)
The so-called “Stretch-Bend Transition” is a vibrational signature that results from the hydrogen-bound OH stretch and the HOH bend of the donor water molecule. This transition is of interest because it is in the near-IR region (the transition is between 5000-5500 cm-1) and it’s intensity pattern is unique. We showed that unlike for the OH stretch transition, the intensity of this two-quanta transition is not dependent on hydrogen-bond strength. This means that this feature has the potential to be used to understand the population of each of these types of hydrogen-bond arrangements in a sample.